|
Strain Name
|
C57BL/6-Cd40tm1(CD40)Bcgen/Bcgen
|
Common Name
|
B-hCD40 mice
|
|
Background
|
C57BL/6
|
Catalog number
|
110009
|
Related Genes
|
CD40 (CD40 antigen)
|
NCBI Gene ID
|
21939
|
Protein expression analysis

Strain specific CD40 expression analysis in homozygous B-hCD40 mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hCD40 (H/H) mice stimulated with anti-CD3ε in vivo (7.5 μg/mice), and analyzed by flow cytometry with species-specific anti-CD40 antibody. Mouse CD40 was exclusively detectable in WT mice. Human CD40 were exclusively detectable in homozygous B-hCD40 but not WT mice.
CD40 in B cells-Spleen

Strain specific CD40 expression analysis in homozygous B-hCD40 mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hCD40 (H/H) mice and analyzed by flow cytometry with species-specific anti-CD40 antibody. Mouse CD40 was detectable in WT mice. Human CD40 were exclusively detectable in homozygous B-hCD40.
CD40 in B cells-Blood

Strain specific CD40 expression analysis in homozygous B-hCD40 mice by flow cytometry. Blood were collected from WT and homozygous B-hCD40 (H/H) mice and analyzed by flow cytometry with species-specific anti-CD40 antibody. Mouse CD40 was detectable in WT mice. Human CD40 was exclusively detectable in homozygous B-hCD40.
CD40 in DC cells-Spleen

Strain specific CD40 expression analysis in homozygous B-hCD40 mice by flow cytometry. DC cells in spleen were collected from WT and homozygous B-hCD40 (H/H) mice and analyzed by flow cytometry with species-specific anti-CD40 antibody. Mouse CD40 was detectable in WT mice. Human CD40 was exclusively detectable in homozygous B-hCD40.
B cells in B-hCD40 mice bind anti-human CD40 antibody

Analysis of splenocytes of B-hCD40 mice by FACS. Splenocytes were isolated from female B-hCD40 mice (n=3). Flow cytometry analysis of the splenocytes was performed to assess human CD40 expression on B cells. Single live cells were gated for CD45 population and used for further analysis as indicated here. Human CD40 expression was detectable on CD19 B cells in B-hCD40 mice as evidenced by selicrelumab (in house) binding vs isotype control.
Analysis of spleen leukocytes cell subpopulations in B-hCD40 mice


Analysis of spleen leukocyte subpopulations by FACS
Splenocytes were isolated from female C57BL/6 and B-hCD40 mice (n=3) Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T, B, NK, Monocyte, DC and macrophage cells in homozygous B-hCD40 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCD40 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen.
Analysis of spleen T cell subpopulations in B-hCD40 mice

Analysis of spleen T cell subpopulations by FACS
Splenocytes were isolated from female C57BL/6 and B-hCD40 mice (n=3). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of CD8, CD4, and Treg cells in homozygous B-hCD40 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCD40 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in spleen.
Blood routine test in B-hCD40 mice

Complete blood count (CBC) Blood from female C57BL/6 and B-hCD40 mice (n=3) were collected and analyzed for CBC. There was no differences among any measurement between C57BL/6 and B-hCD40 mice, indicating that introduction of hCD40 in place of its mouse counterpart does not change blood cell composition and morphology.
Blood chemistry of B-hCD40 mice

Blood chemistry tests of B-hCD40 mice. Serum from the C57BL/6 and B-hCD40 mice (n=3) was collected and analyzed for levels of ALT (alanine aminotransferase) and AST (aspartate aminotransferase). There was no differences on either measurement between C57BL/6 and B-hCD40 mice, indicating that introduction of hCD40 in place of its mouse counterpart does not change ALT and AST levels or health of liver.
In vivo efficacy of anti-human CD40 antibodies

Antitumor activity of anti-human CD40 antibodies in B-hCD40 mice. (A) Anti-human CD40 antibodies inhibited MC38 tumor growth in B-hCD40 mice. Murine colon cancer MC38 cells (5ⅹ105) were subcutaneously implanted into heterozygous B-hCD40 mice (female, 4 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with three anti-human CD40 antibodies with doses and schedules indicated in panel (B) Body weight changes during treatment. As shown in panel A, anti-human CD40 antibodies were efficacious in controlling tumor growth in B-hCD40 mice, demonstrating that the B-hCD40 mice provide a powerful preclinical model for in vivo evaluation of anti-human CD40 antibodies. Values are expressed as mean ± SEM
In vivo efficacy of anti-human CD40 antibodies

Antitumor activity of anti-human CD40 antibody in B-hCD40 mice. (A) Anti-human CD40 antibody inhibited MC38 tumor growth in B-hCD40 mice. Murine colon cancer MC38 cells(5ⅹ105) were subcutaneously implanted into homozygous B-hCD40 mice (female,4 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human CD40 antibody with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human CD40 antibody was efficacious in controlling tumor growth in B-hCD40 mice, demonstrating that the B-hCD40 mice provide a powerful preclinical model for in vivo evaluation of anti-human CD40 antibodies. Values are expressed as mean ± SEM.
In vivo efficacy of anti-human CD40 antibodies

Antitumor activity of anti-human CD40 antibody in B-hCD40 mice. (A) Anti-hCD40 antibody Selicrelumab (in house) inhibited MC38 tumor growth in B-hCD40 mice. Murine colon cancer MC38 cells(5ⅹ105) were subcutaneously implanted into homozygous B-hCD40 mice (female,8-9 week-old, n=5). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with anti-human CD40 antibody Selicrelumab (in house) with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human CD40 antibody Selicrelumab (in house) was efficacious in controlling tumor growth in B-hCD40 mice, demonstrating that the B-hCD40 mice provide a powerful preclinical model for in vivo evaluation of anti-human CD40 antibodies. Values are expressed as mean ± SEM.
High dose of selicrelumab (anti-human CD40) moderately resulted in body weight decrease in B-hCD40 mice but not in wild type C57BL/6 mice

High does of selicrelumab (anti human CD40) moderately reduced body weight in B-hCD40 mice but not in wild type C57BL/6 mice. Homozygous B-hCD40 and C57BL/6 mice were treated with vehicle or selicrelumab (in house) on days indicated by red arrows (n=3). Selicrelumab at 20 mg/kg caused significant body weight reduction compared with vehicle control in B-hCD40 mice, while the same treatment had no effect in C57BL/6 mice. Values are expressed as mean ± SEM.
Selicrelumab Analog Toxicity Evaluation

High-dose human CD40 antibody caused significant changes in AST and ALT in B-hCD40 mice. Homozygous B-hCD40 and C57BL/6 mice were treated with PBS or selicrelumab (in house) as indicated on the Dosing Schedule (n=3). The blood biochemical analysis was conducted on B-hCD40 mice treated with anti-human CD40 antibody 24 hours after the termination of efficacy evaluation experiment. Selicrelumab at 20 mg/kg caused significant increase of AST and ALT compared with PBS control in B-hCD40 mice, while the same treatment had no effect in C57BL/6 mice. Values are expressed as mean ± SEM.
Selicrelumab treatment resulted in reduction of number of RBC and hemoglobin content

High-dose human CD40 antibody caused significant changes in complete blood count in B-hCD40 mice. Homozygous B-hCD40 and C57BL/6 mice were treated with PBS or selicrelumab (in house) as indicated on the graph (n=3). The complete blood count (CBC) was performed 24 hours after last dosing on day 11. Selicrelumab at 20 mg/kg caused significant reduction of WBC, RBC, Hb, HCT and LY#, and increase of RDW and MPV compared with PBS control in B-hCD40 mice, while the same treatment had no effect in C57BL/6 mice. Values are expressed as mean ± SEM.
High dose of selicrelumab therapy resulted in increased lymphocyte infiltration in liver and kidney in B-hCD40 mice but not in C57BL/6 mice

High dose selicrelumab caused liver and kidney pathological changes in B-hCD40 mice. Homozygous B-hCD40 and C57BL/6 mice were treated with PBS or selicrelumab (n=3). The tissue pathology analysis was performed on day 14. Selicrelumab at 20 mg/kg caused increased lymphocyte infiltration (as indicated by arrows) into liver and kidney in B-hCD40 mice, while the same treatment had no effect in C57BL/6 mice.
In vivo efficacy of anti-human CD40 antibody with CIA model
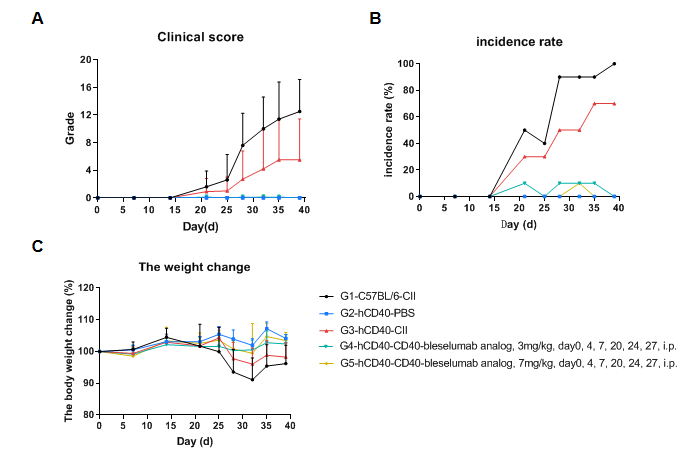
Efficacy of the anti-human CD40 antibody in a mouse arthritis model. (A) Clinical score; (B) Incidence of disease; (C) Change in mouse body weight. The results showed that in the modeling group (G1&G3), the clinical scores of the mice significantly increased, indicating successful establishment of the CIA model, while body weight fluctuations were more pronounced compared to the non-model group (G2). After the administration of anti-human CD40 antibody (bleselumab analog, synthesized in-house), the average clinical score of the treatment group (G4&G5) was significantly lower than that of the model group, suggesting that the condition of mice was effectively controlled. Additionally, the maximum incidence in the treatment group also showed significantly lower than the model group, indicating that the anti-human CD40 antibody has a therapeutic effect on this disease.
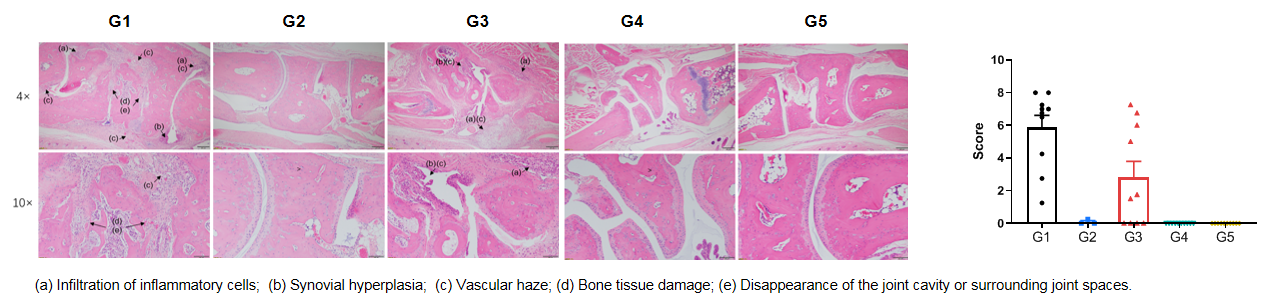
The pathological analysis of the efficacy of the anti-human CD40 antibody bleselumab (in-house) in CIA model. (A) Pathological sections stained with H&E; (B) Pathological scores. The results showed that in the non-model group (G2), there were no significant abnormal changes observed, with a smooth cartilage surface in the ankle joint and a clearly defined joint cavity. In the modeling group (G1&G3), the animals exhibited the infiltration of inflammatory cells (a), synovial hyperplasia (d) and vascular haze (e). Compared to the model group, the bleselumab treatment groups (G4&G5) significantly alleviated the disease symptoms in the mice. This suggests that bleselumab has a therapeutic effect on arthritis in mice.
































 京公网安备:
京公网安备: