创新动物模型赋能新药研发
齐全的动物/细胞品系——3100+品系
完整的表型分析和药效验证数据
规模化稳定供应能力——年供应80万只
独立知识产权,为药物研发保驾护航
工业化生产,动物体重均一性控制,每批动物可追溯
AAALAC全资质认证、国际SPF等级


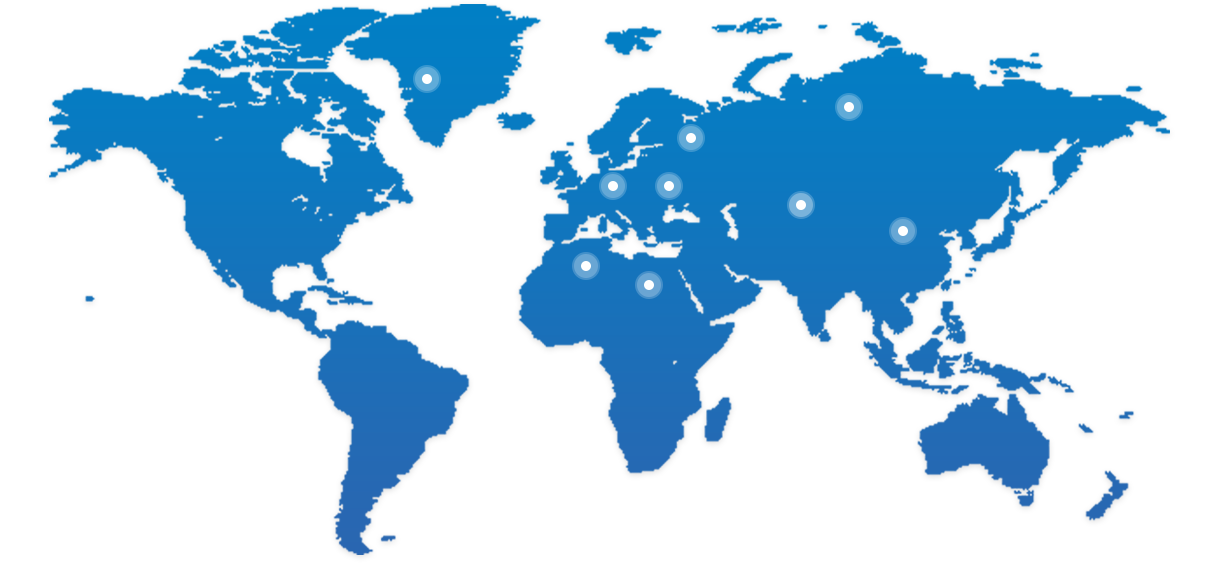
BioMice已经从中国走向全球
我们愿与合作伙伴资源共享,共同推进新药研发
美国
加拿大
德国
瑞典
法国
英国
荷兰
西班牙
俄罗斯
比利时
波兰
丹麦
芬兰
瑞士
挪威
葡萄牙
意大利
中国
韩国
日本
印度
沙特阿拉伯
以色列
新加坡
















 京公网安备:
京公网安备: